Training Courses
 The RESILIENCE partners offer a range of training courses and outreach events across the UK and throughout the year. Most courses are run to regularly updated advertised dates but we also welcome enquiries about in-house provision of customised training which can be provided at a date and place to match your needs. Self-paced Online Training opportunities can be found here.
The RESILIENCE partners offer a range of training courses and outreach events across the UK and throughout the year. Most courses are run to regularly updated advertised dates but we also welcome enquiries about in-house provision of customised training which can be provided at a date and place to match your needs. Self-paced Online Training opportunities can be found here.
You can filter the course list below to find a course by clicking on a Category or Tag button of interest to you.
Training Courses available from RESILIENCE
BioProcess360: Integrated Upstream & Downstream Bioprocessing training

This 5-day comprehensive training course offers an end-to-end exploration of the biomanufacturing process, integrating both upstream and downstream operations into a unified, hands-on learning experience. Participants will begin by cultivating a recombinant protein using a microbial expression system in a 30 L stainless steel stirred-tank bioreactor, gaining direct experience in bioreactor setup, sterilisation, inoculation, monitoring, and harvest procedures.
Certificate of Upstream Bioprocessing
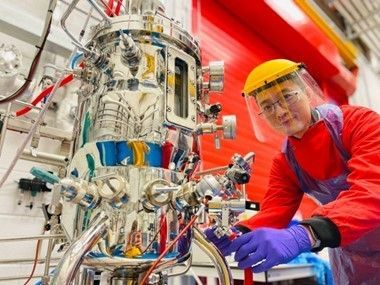
The course is aimed at early career professionals and anyone who wants to develop an understanding of upstream biomanufacturing techniques and processes. This 3-day course consists of six lectures, enhanced with practical demonstrations using bench-scale, pilot-scale, and single-use bioreactors. In particular, attendees will gain hands-on fermentation experience using a 30L-scale, stainless steel, stirred tank bioreactor.
Design of Experiments for Bioprocess Optimisation

Learn how experimental design techniques can be used to rapidly understand and optimise bioprocess performance.
Flow Cytometry for Advanced Therapies

In this advanced course, trainees are introduced to the underlying principles behind the use of flow cytometry in advanced therapies research/manufacture. Learning achieved by lectures, classroom activities and hands-on laboratory sessions.
Introduction to GMP and Quality in Pharmaceutical Manufacturing

Aimed at anyone with an interest in working in medicines manufacturing, this popular course provides an essential introduction to GMP (Good Manufacturing Practice), Data Integrity and ALCOA+ principles, Method and Process Validation, and much more.
Measuring and Documenting Quality, Risks and Failures in GMP
Learn how quality, risks, and failures are measured and documented in GMP medicines manufacturing. Over this two-day course you will learn how to apply Quality by Design, identify key quality and process factors, and set up strong Quality Management Systems to ensure safe, consistent products.
Pilot-Scale Upstream Bioprocessing: 300L Bioreactor training

This 4-day intensive training course is designed for professionals and researchers looking to advance their expertise in large-scale upstream bioprocessing, with a focus on scale-up to 300 L pilot-scale operations. Participants will explore the principles and practical steps involved in scaling microbial cell culture to production-relevant volumes, supported by expert-led lectures, live demonstrations, and hands-on training.
Principles of Fermentation Processes

Focusing on how to specify the process and the fundamentals for characterisation, this module will strengthen your knowledge of fermentation processes and through a series of lectures and case studies.
Quality by Design for Effective Bioprocess Characterisation and Validation

Get expert guidance on choosing on how best to integrate QbD, DoE and PAT into lifecycle approach to process characterisation and operation of lab-scale microbial bioreactors. 4-days of training featuring a series of stimulating interactive lectures from experts in the field and teamwork-based activities.
