Training
The RESILIENCE partners offer a range of training courses and outreach events across the UK and throughout the year. Forthcoming scheduled training dates are shown below. You can also:
Browse our full range of Training Courses
Access self-paced, on-demand online training
Join our unique Leadership Accelerator Programme
Forthcoming Dates
Introduction to GMP and Quality in Pharmaceutical Manufacturing

Darlington
Aimed at anyone with an interest in working in medicines manufacturing, this popular course provides an essential introduction to GMP (Good Manufacturing Practice), Data Integrity and ALCOA+ principles, Method and Process Validation, and much more.
Quality by Design for Effective Bioprocess Characterisation and Validation

London
Get expert guidance on choosing on how best to integrate QbD, DoE and PAT into lifecycle approach to process characterisation and operation of lab-scale microbial bioreactors. 4-days of training featuring a series of stimulating interactive lectures from experts in the field and teamwork-based activities.
Certificate of Upstream Bioprocessing
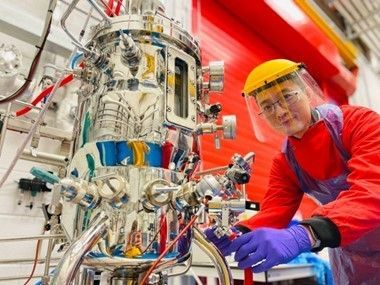
Edinburgh
The course is aimed at early career professionals and anyone who wants to develop an understanding of upstream biomanufacturing techniques and processes. This 3-day course consists of six lectures, enhanced with practical demonstrations using bench-scale, pilot-scale, and single-use bioreactors. In particular, attendees will gain hands-on fermentation experience using a 30L-scale, stainless steel, stirred tank bioreactor.
BioProcess360: Integrated Upstream & Downstream Bioprocessing training

Edinburgh
This 5-day comprehensive training course offers an end-to-end exploration of the biomanufacturing process, integrating both upstream and downstream operations into a unified, hands-on learning experience. Participants will begin by cultivating a recombinant protein using a microbial expression system in a 30 L stainless steel stirred-tank bioreactor, gaining direct experience in bioreactor setup, sterilisation, inoculation, monitoring, and harvest procedures.
Pilot-Scale Upstream Bioprocessing: 300L Bioreactor training

Edinburgh
This 4-day intensive training course is designed for professionals and researchers looking to advance their expertise in large-scale upstream bioprocessing, with a focus on scale-up to 300 L pilot-scale operations. Participants will explore the principles and practical steps involved in scaling microbial cell culture to production-relevant volumes, supported by expert-led lectures, live demonstrations, and hands-on training.
