Training
The RESILIENCE partners offer a range of training courses and outreach events across the UK and throughout the year. Forthcoming scheduled training dates are shown below. You can also:
Browse our full range of Training Courses
Access self-paced, on-demand online training
Join our unique Leadership Accelerator Programme
Forthcoming Dates
Introduction to GMP and Quality in Pharmaceutical Manufacturing

Darlington
Aimed at anyone with an interest in working in medicines manufacturing, this popular course provides an essential introduction to GMP (Good Manufacturing Practice), Data Integrity and ALCOA+ principles, Method and Process Validation, and much more.
Applied Skills in Mammalian Cell Bioprocessing

Edinburgh
This hands-on course teaches laboratory scientists and engineers how to handle and maintain mammalian cells applying appropriate aseptic techniques as well as how to evaluate cell quality using a range of analytical assays.
Certificate of Upstream Bioprocessing
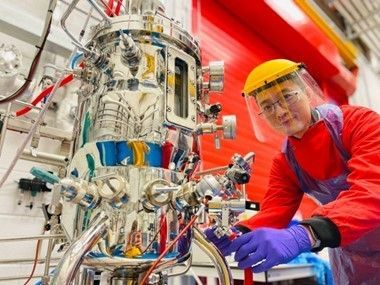
Edinburgh
The course is aimed at early career professionals and anyone who wants to develop an understanding of upstream biomanufacturing techniques and processes. This 3-day course consists of six lectures, enhanced with practical demonstrations using bench-scale, pilot-scale, and single-use bioreactors. In particular, attendees will gain hands-on fermentation experience using a 30L-scale, stainless steel, stirred tank bioreactor.
Cell and Gene Therapy Bioprocessing and Manufacture

London
Cell and Gene Therapy is fast becoming an established field that aims to utilize the power of cell-based medicines to deliver new therapies for the 21st Century. However, translating the discovery science into manufacturable products has so far been challenging and so multi-disciplinary engineers who possess unique skills are urgently needed to address the manufacture of these complex medicinal products. This course addresses the real-world challenges of translating discoveries to the clinic in a way that is commercially viable.
BioProcess360: Integrated Upstream & Downstream Bioprocessing training

Edinburgh
This 5-day comprehensive training course offers an end-to-end exploration of the biomanufacturing process, integrating both upstream and downstream operations into a unified, hands-on learning experience. Participants will begin by cultivating a recombinant protein using a microbial expression system in a 30 L stainless steel stirred-tank bioreactor, gaining direct experience in bioreactor setup, sterilisation, inoculation, monitoring, and harvest procedures.
Pilot-Scale Upstream Bioprocessing: 300L Bioreactor training

Edinburgh
This 4-day intensive training course is designed for professionals and researchers looking to advance their expertise in large-scale upstream bioprocessing, with a focus on scale-up to 300 L pilot-scale operations. Participants will explore the principles and practical steps involved in scaling microbial cell culture to production-relevant volumes, supported by expert-led lectures, live demonstrations, and hands-on training.
Advanced Downstream Bioprocessing: From Harvest to Purity

Edinburgh
This 3-day hands-on training course is designed for professionals and researchers looking to develop expertise in downstream bioprocessing, covering key techniques such as Disc-stack Centrifugation, High-pressure Homogenization, Tangential Flow Filtration (TFF), and Chromatography.
Manufacturing Technologies for ATMP Production

Birmingham
This course, delivered in partnership with Cytiva, will examine process technologies and their operation for end-to-end ATMP production.
