Cell & Gene Therapy Bioprocessing & Manufacture
Self-paced online for up to 9 months
7.5 course credits
£950
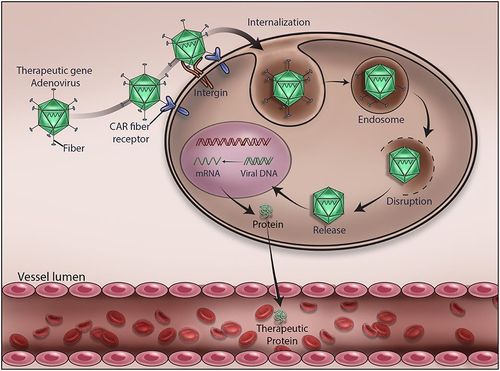 Online, self-paced course focusing on the development of gene therapeutics with emphasis on viral delivery, GMP, process and facility design as well as manufacture and commercialisation.
Online, self-paced course focusing on the development of gene therapeutics with emphasis on viral delivery, GMP, process and facility design as well as manufacture and commercialisation.
The aim of the course is to provide learners with a comprehensive and up-to-date development of the gene therapy area with specific reference to the development, manufacturing and commercialisation of gene therapies. Topics are addressed using a combination of lectures, training and animation videos, and an assessed quiz.
Learning outcomes:
Identify diseases where gene therapies re applicable
Understand key elements in virology to create gene therapies
Identify potential application of stem cells to treat diseases
Understand ethical and regulatory implications of gene therapies
Identify and recognise challenges in gene therapy development and manufacture
Recommended for:
The course is designed for engineers who would like to pursue a career in gene therapy as well as life scientists and clinicians working in the field of regenerative medicine and cell and gene therapy. It will also be particular interest to project managers, funders and policy makers wanting to gain an understanding in this field. It also provides a fundamental base for life scientists and engineers from biopharma who wish to repurpose skills for the cell and gene therapy sector. By attending the course, the learner will network with sector leaders and subject matter experts (from academia and industry) and also increase the learners’ creativity and professional status.
Interested in online learning? You may also like UCL’s self-paced Industrial Biotechnology: Sustainable Bioprocesses and Biorefineries MBI module.
